Principles of friction welding
- Top
- Business information
- Machine tool business
- Technical information
- Friction welding machines
- Understand friction welding
- Principles of friction welding
It is widely known that heat is produced when objects rub together. Man has known of the heat produced by friction since primitive times. Friction welding is one example of applied technology used to bond metals together using this heat.
Basic friction welding cycle
![[1] Slide advance](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_001.jpg)
[1] Slide advance
![[2] Spindle rotation](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_002.jpg)
[2] Spindle rotation
![[3] Blank contact](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_003.jpg)
[3] Blank contact
![[4] Frictional heat generation](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_004.jpg)
[4] Frictional heat generation
![[5] Upset pressure application](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_005.jpg)
[5] Upset pressure application
![[6] Slide retract, workpiece unloading](https://www.en.izumi-mfg.co.jp/hubfs/image/business/machine/technology/fw/knowledge/mechanism/img_mechanism_006.jpg)
[6] Slide retract, workpiece unloading
Frictional heat generation process
By rotating materials and pushing them together under constant pressure (friction pressure), the temperature of the surface being bonded rises due to frictional heat, and high-temperature layers are formed.
Upset pressure application process
Rotation is then stopped abruptly, and even higher pressure (upset pressure) is applied. This condition is maintained for a fixed length of time, and solid state bonding is performed under high temperature and high pressure.
The two base materials are securely bonded by interatomic attraction*, and the tensile strength ends up even stronger than the base material.
* What is interatomic attraction (Van der Waals force)?
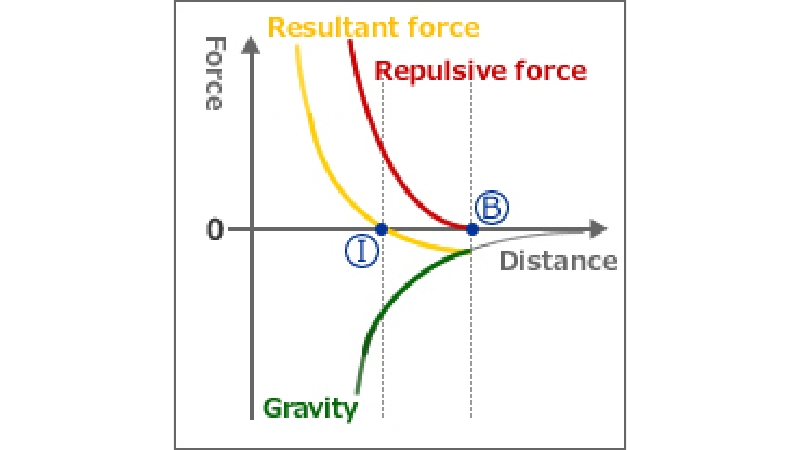
[1] Inter-atomic distance of two base materials, whose deformation resistance has been reduced due to softening caused by frictional heat, narrows.
[2] By bringing position (B) at which the “attractive force” exerted by the atoms in the material surface during contact acts close to position (I) where this attraction and the repelling “repulsive force” are in a state of equilibrium, bonding is completed.
[3] The formation of a state of equilibrium is possible at or below the melting point of the metal.
[4] Bonding is performed even between dissimilar metals if it is possible to form a state of equilibrium between attractive force and repulsive force.


